The Shorter a Wave's Wavelength the Greater Its Energy
Move parallel to the direction of travel. Higher rates of velocity change result in higher frequency shorter wavelength radiation.
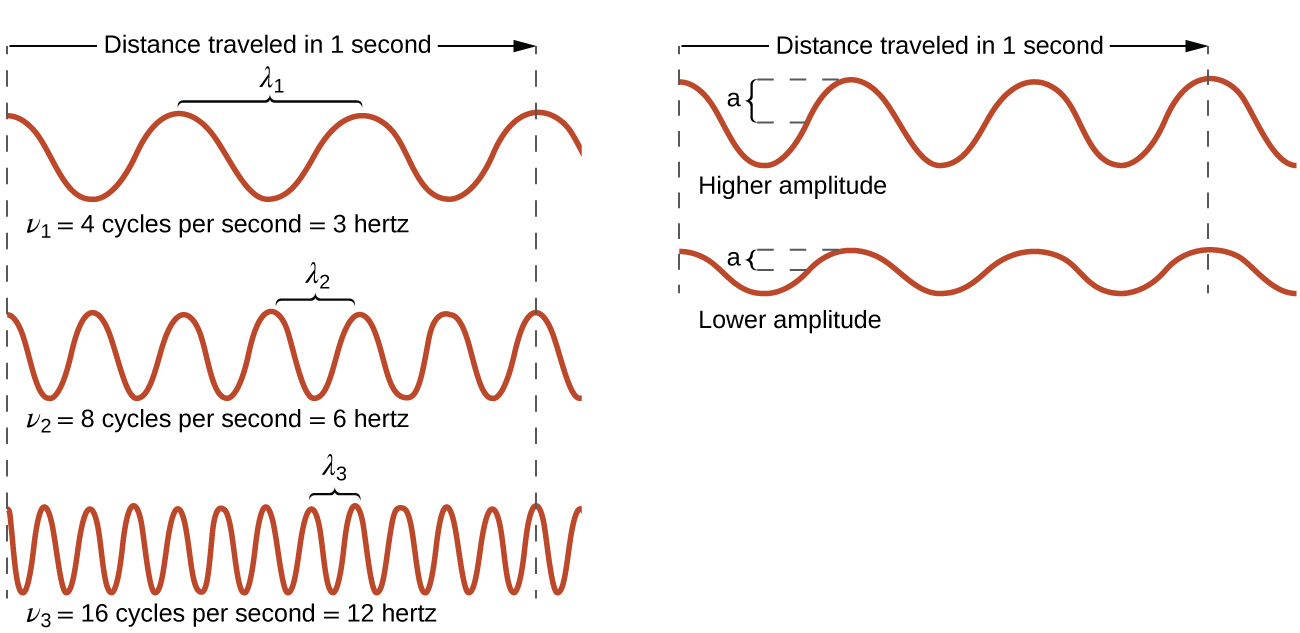
6 1 Electromagnetic Energy Chemistry
Solution a The lower wave has a longer wavelength greater distance between peaks.
. Now shorter the wavelength greater is the frequency of light also which correlates directly with the energy of the photon. The Planck law gives a. The energy content of a photon is often expressed in terms of the unit electron-volt or eV.
Photons associated with X-rays and gamma rays which have very high. The shorter is its wavelength. As a result the lower the energy the longer the wavelengths and the lower the frequency.
The lower is its speed. The shorter the wavelength of a wave the higher its energy. As a result the photons wavelength is also connected to its energy.
In a transverse wave the individual particles of the medium _____. How is the wavelength of an EM wave related to its energy. Similarly a photon wave with a longer wavelength will have a lower frequency and thus less energy.
The shorter the wavelength of visible light the higher the frequency and the greater the energy of the photons. The equation E hv explains why shorter wavelengths have more energy than longer wavelengths. It is an inverse relationship.
The higher the frequency of an electromagnetic wave and the shorter its corresponding wavelength the greater will be the energy of a photon associated with it. TRUE T or F. Hence the higher the frequency the shorter the wavelength and the higher the energy of the wave.
The longer the wavelength the higher its energy. The shorter the wavelength of light the bluer the color. The longer the wavelength of a wave the less it affects energy.
This range is. The greater the energy the shorter the wavelengths and higher the frequency. Also the higher is the radiations received by the object the more it will emit out depending upon the emissivity factor of the object.
The shorter the wavelength X-rays gamma rays the greater the energy. Green light has a short wavelength and its bright because it carries much energy per unit area. The longer the wavelength.
The photon waves wavelength contains information about its energy. A shorter wavelength photon wave will have a higher frequency and consequently higher energy. Frequency is always inversely related to the wavelength.
The gamma rays x-rays and UV rays have a very short wavelength hence the energy of these waves is very high compared to visible infrared microwaves or radio waves. The greater is its amplitude. The shorter the wavelength the lower its energy.
Wavelength will become shorter. E hn is the energy equation. The Planck Radiation Law gives the intensity of radiation as a function of wavelength for a fixed temperature.
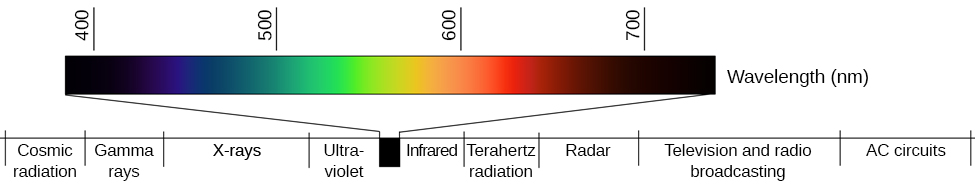
Shorter the wavelength greater is the energy of the photon. The energy associated with a wave is directly proportional to its frequency. The expanded visible -light portion of Figure 64 tells you that red light has a longer wavelength than blue light.
The longer the wavelength of a wave the higher its energy. This new false-colored image from NASAs Hubble Chandra and Spitzer space telescopes shows a giant jet of particles that has been shot out from. Shorter the wavelength the higher the frequency of the electromagnetic wave and greater is the energy of the waveform or the photon.
Red light blue light and purple light have even longer wavelengths and are also very bright. The longer is its period. The longer the wavelength the lower its energy.
Accordingly how does changing the frequency affect the energy of a wave. The bluer the light the more energy it has and things like gamma rays and X-rays have more energy still than anything our eyes can detect. Longer waves can carry more energy per unit area than shorter ones so theyre usually more powerful when exposed to the same amount of sunlight.
What happens when a frequency of a wave increases. Shorter wavelengths equate to higher frequency due to the c vlambda equation and frequency v is directly proportional to the energy of a photon. The shorter the wavelength the higher its energy.
Spectral lines are produced when an electron makes a transition from one energy state to another. The lower wave has the longer wavelength lower frequency and would be the red light. The shorter the wavelength of a wave the lower its energy.
The shorter a waves wavelength the greater its energy. The higher the frequency of the wave is _____. Energy is connected to wavelength and frequency in the same way that wavelength and frequency are related to light.
The observed intensity of thermal radiation emitted by as a function of wavelength can be described by the Planck Radiation Law Physics 221. Figure 52 shows the wide range of electromagnetic radiation from AM radio waves with a wavelength of 10 4 m to gamma waves with a wavelength of 10-12 m. How does energy of the EM waves relate to their wavelength.
Energy is inversely proportional to wavelength. The shorter the wavelength of visible light means what.

Waves What Is Considered A Wavelength Physics Stack Exchange
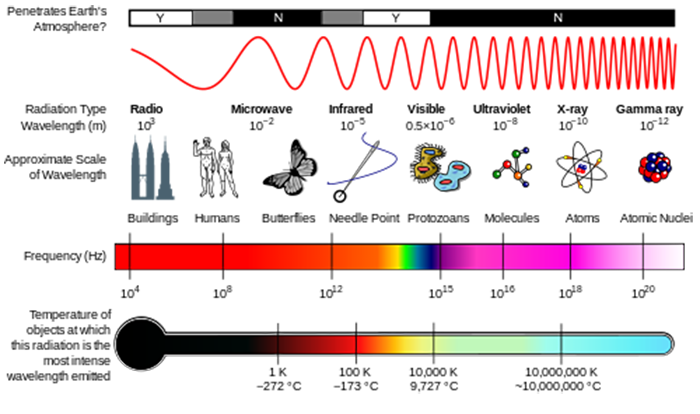
6 3 How Is Energy Related To The Wavelength Of Radiation Meteo 300 Fundamentals Of Atmospheric Science
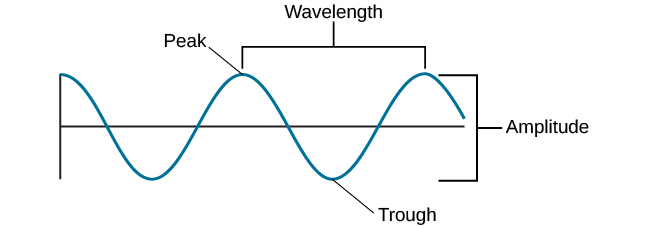
Waves And Wavelengths Introduction To Psychology

Electromagnetic Waves Different Waves Different Wavelengths
What Is Difference Between Radiation And Wavelength Quora

Shorter Wavelength An Overview Sciencedirect Topics
Why Do Short Wavelengths Have More Energy Than Long Wavelengths Quora
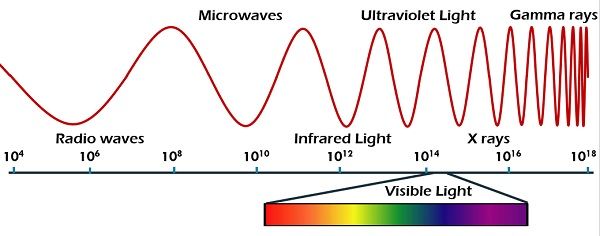
Difference Between Radio Wave And Microwave With Comparison Chart Tech Differences

Light Properties Principles Of Structural Chemistry
What Happens As The Wavelength Of A Wave Decreases Quora
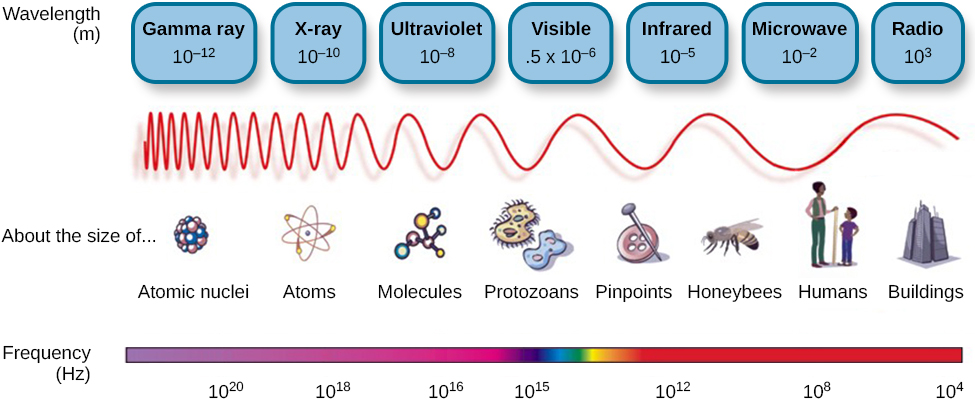
Waves And Wavelengths Introduction To Psychology
Why Do The Wavelengths Of Electromagnetic Radiation Get Shorter When Their Frequencies Get Higher Quora
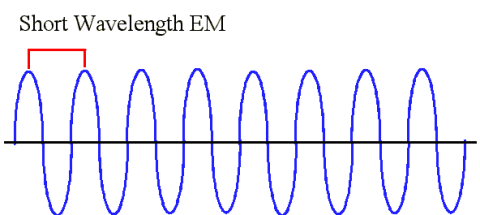


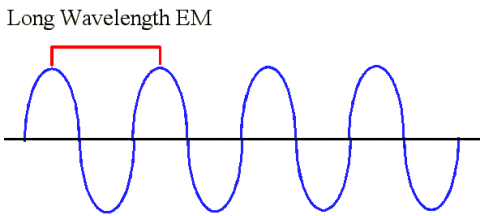
Comments
Post a Comment